Drug Discovery Recharge Unit: Seahorse and Octet Instruments
Seahorse
UC customers: $229/assay
DARTs/NonProfits: $306/assay
External/For Profit: $351/assay
For questions or to get started, please contact unit director Alexey Tomilov, PhD, at atomilov@ucdavis.edu.
Or call 530-902-1051
Equipment Description and Usage Service
The Seahorse XF flux analyzer equipment is available for recharge use at the UC Davis. The 24-well Seahorse system provides researchers the opportunity to perform medium-throughput, real-time measurement of mitochondrial function and cellular bioenergetics in an expedited manner compared to traditional techniques. Seahorse XF Analyzers measure oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of live cells in a multi-well plate, interrogating key cellular functions such as mitochondrial respiration and glycolysis. XF Analyzers perform compound addition and mixing, label-free analytical detection, and automatic measurement of OCR and ECAR in real time (https://www.agilent.com/en/products/cell-analysis/seahorse-analyzers).
Seahorse XF24 Analyzer
Seahorse XFe24 Analyzers measure OCR and ECAR of live cells in a 24-well plate format. OCR and ECAR rates are key indicators of mitochondrial respiration and glycolysis and these measurements provide a systems-level view of cellular metabolic function in cultured cells.
- Real-time Results- this integrated system reports metabolic rates in just minutes, without sample extraction or labeling. Wave software controls the instrument and performs rate measurements on the fly for same day results.
- Live-Cell Responses – detect responses to substrates, inhibitors, and other compounds in real time via the 4-port injection system and automated mixing while maintaining physiologic temperature (37oC).
- Sensitive- analyze as few as 10,000 cells per well in the custom 24-well plate. Cell number requirements vary by cell type; consult the Cell Reference Database for details. The 24-well microplate and system has capacity for larger and/or more metabolically active samples than the 96-well system.
- Compatible with a variety of samples due to the precision-controlled heating tray, which can maintain 16-42oC (12oC-20oC above ambient conditions).
- Analyze function of islet or other mobile samples with the Seahorse XF24 Islet Capture Plate
- Measure mitochondrial function with the Seahorse XF Cell Mito Stress Test
- Generate a metabolic phenotype within one hour with the Seahorse XF Cell Energy Phenotype Test
- Measure glycolytic rates in live cells with the Seahorse XF Glycolytic Rate Assay
- Quickly determine dependency of cellular energy production on mitochondrial substrates with the Seahorse XF Mito Fuel Flex Test
- Easily create assay protocols and analyze data.
- For Research Use Only. Not for use in diagnostic procedures.
Octet® Real-Time Drug and Protein Binding Kinetics Unit
UC customers: $129/hr
DARTs/NonProfits: $171/hr
External/For Profit: $250/hr
For questions, and to reserve machine time, please contact unit director Alexey Tomilov, PhD, at atomilov@ucdavis.edu, or call 530-902-1051.
Equipment Description and Usage Service
The ForteBio Octet® RED384 equipment is available for recharge use at the UC Davis Octet® Real-Time Drug and Protein Binding Kinetics Unit.The Octet system provides researchers the opportunity to perform high-throughput, real-time, drug-protein and protein-protein binding kinetics in an expedited manner compared to traditional techniques. This machine is a relatively new technological advancement and is the only one available in Northern California except UCSF.
The Octet® system provides many advantages over traditional methods for detecting drug- protein and protein-protein interactions. The high-throughput aspect of the system means that a library of small molecules or known proteins can be screened for interaction with a protein of interest both quickly and at large scale. The BioLayer Interferometry (BLI) Technology provides real-time protein-small molecule or protein-protein association and dissociation kinetics. The high sensitivity of the assay means that much lower protein amounts are required for analysis compared to traditional immunoprecipitation, NMR, or ELISA methods. In addition, crude protein extracts may be used, and the Octet® system has considerable flexibility to work with both labeled and non-labeled protein targets.
Use charges for the Octet: Users buy biosensors and plates, and pay for machine time. Users order their tips from Forte Bio, from this list https://www.fortebio.com/biosensor-types.html and pay for them with a credit card or UCD Purchase Order number. Users need to order plates from Forte Bio (384 well), Part number 18-5076 (Case) or 18-5080 (Pack of 10 plates, ~$15/plate). To order 96-well plates, these are ordered from greiner bio-one, Greiner Bio-One: GBO (grainer bio-one, MICROPALTE 96 WELL, PS, F-BOTTOM (CHIMNEY WELL) BLACK, NON-BINDING, 10 PCS/BAG. REF 655900).
ForteBio Octet® RED384 Instrument
http://www.fortebio.com/bli-technology.html
Bio-Layer Interferometry (BLI) Technology
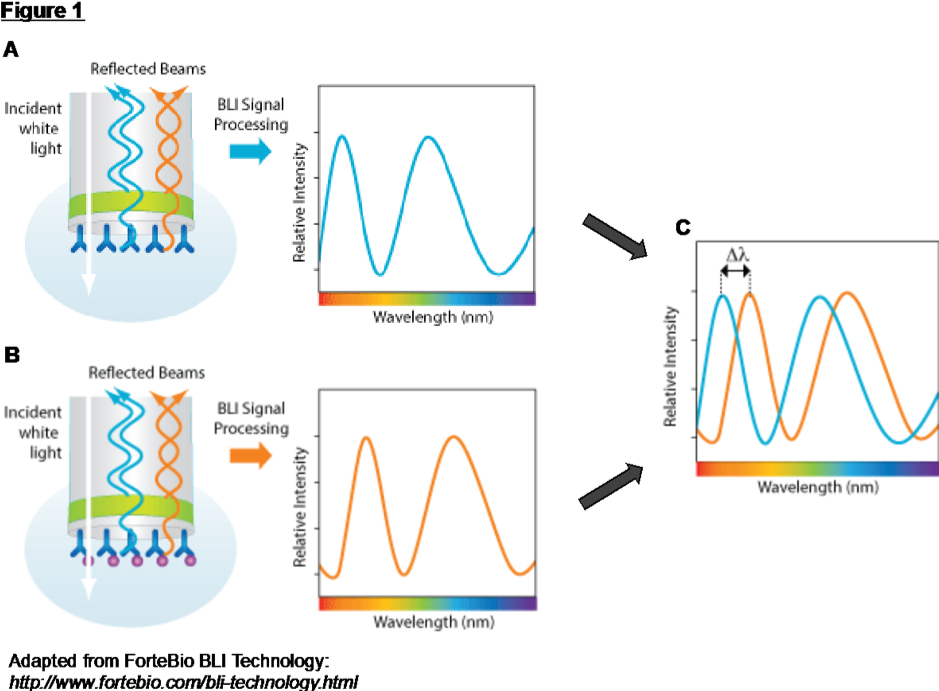
BLI is an optical analytical technique that measures interference patterns between waves of light (Figure 1). White light is directed down a fiber-optic biosensor towards two interfaces: an internal reference layer (Figure 1A), and a biocompatible layer on the surface of the tip (Figure 1B). A thin layer at the tip of the fiber separates these interfaces. Light reflects from each of the two layers, and the reflected beams interfere constructively or destructively at different light spectrum wavelengths; a CCD array detector measures this interference pattern.
Target molecules bind to the two-dimensional coated surface when the tip of a biosensor is dipped into a sample. This molecular layer increases in thickness when more target molecules bind to the surface. As the thickness of the layer increases at the biosensor tip, the effective distance between the two reflective layers increases, creating a shift in the interference pattern of reflected light (Figure 1C). The spectral pattern of the reflected light therefore changes as a function of the molecular layer optical thickness, i.e. the number of molecules bound to the biosensor surface. This spectral shift is monitored at the detector and reported as a change of wavelength (Response [nm] shift) on the Octet® sensorgram.

ForteBio Octet® RED384 Instrument Software
Please visit the Octet® RED384 manufacturer website for information regarding available data analysis software packages:
http://www.fortebio.com/octet-software.html
