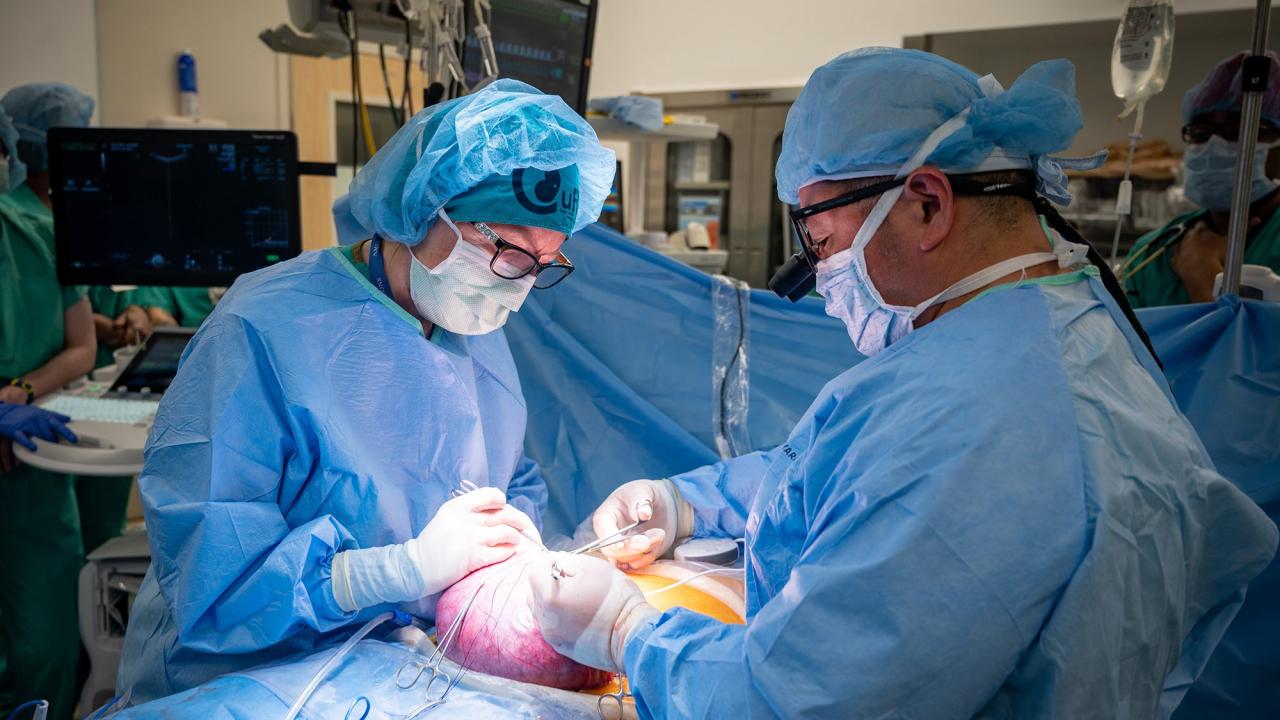
Clinical Trial Offers Hope for Spina Bifida Cure
Interdisciplinary Research Leads to Historic Clinical Trial Delivering Stem Cells During Fetal Surgery to Treat Spina Bifida
When Emily and her husband, Harry, learned that they would be first-time parents, they never expected any pregnancy complications.
But after an 18-week anatomy ultrasound, the Austin, Texas, couple got some difficult news: Their developing child had spina bifida. That same day, they also learned about the CuRe trial, the world’s first spina bifida treatment combining fetal surgery with stem cells at UC Davis Health.
For Emily, it was a lifeline they couldn’t refuse.
“We didn’t know what spina bifida was before we got our diagnosis. We are so thankful that we got to be a part of this. We are giving our daughter the very best chance at a bright future.”
— Emily
Emily enrolled in the trial, and her developing baby was the world’s first to receive the groundbreaking treatment. This was made possible by a landmark clinical trial that was the result of more than two decades of research into the use of human stem cells in fetal surgery and animal modeling. The one-of-a-kind treatment, delivered while a fetus is still developing in the mother’s womb, could improve outcomes for children with this birth defect.
Launched in the spring of 2021, the clinical trial is known formally as the “CuRe Trial: Cellular Therapy for In Utero Repair of Myelomeningocele.” Thirty-five patients will be treated in total.
The four babies from the trial that have been born so far will be monitored by the research team until 30 months of age to fully assess the procedure’s safety and effectiveness.
The first phase of the trial is funded by a $9 million state grant from the state’s stem cell agency, the California Institute for Regenerative Medicine, or CIRM.
Improving Lives for Animals and Humans: an extraordinary story of the collaboration between UC Davis Schools of Medicine, Veterinary Medicine and College of Engineering
“This clinical trial could enhance the quality of life for so many patients to come,” said Emily, who traveled from Austin to participate. Her daughter, Robbie, was born in September 2021.
This experience has been larger than life and has exceeded every expectation. I hope this trial will enhance the quality of life for so many patients to come. ”
“This experience has been larger than life and has exceeded every expectation. I hope this trial will enhance the quality of life for so many patients to come. We are honored to be part of history in the making,” Emily said.
Spina bifida, also known as myelomeningocele, occurs when spinal tissue fails to fuse properly during the early stages of pregnancy. The birth defect can lead to a range of lifelong cognitive, mobility, urinary and bowel disabilities. It affects 1,500 to 2,000 children in the U.S. every year. It is often diagnosed through ultrasound.
