 Equine Protozoal Myeloencephalitis (EPM)
Equine Protozoal Myeloencephalitis (EPM)
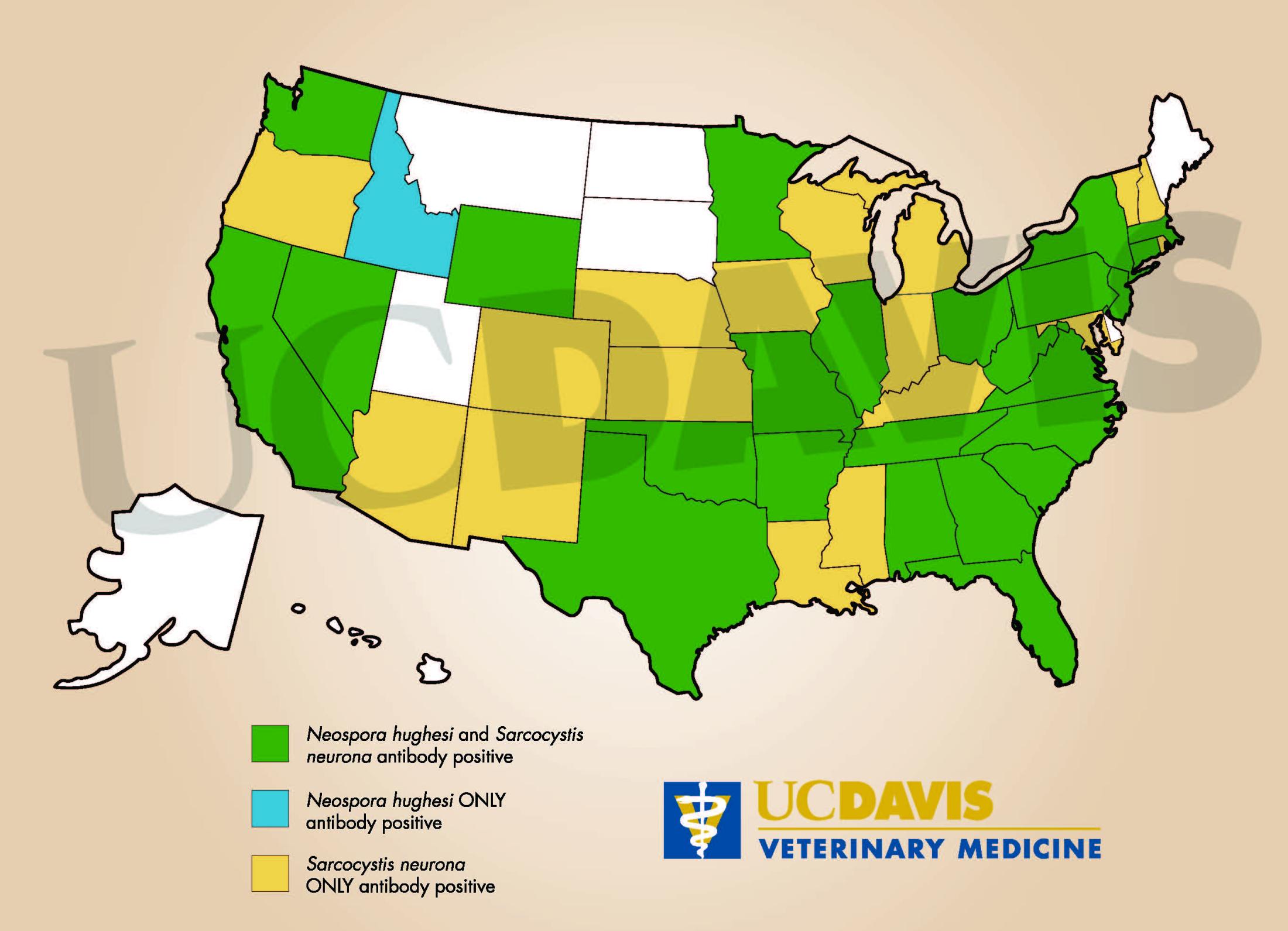
SarcoFluor™ and NeoFluor™ provide quantitative titers and likelihood ratios for Equine Protozoal Myeloencephalitis (EPM), supporting the diagnosis of infection with Sarcocystis neurona and Neospora hughesi, respectively.
If serum and CSF are submitted simultaneously for SarcoFluor™ or NeoFluor™ testing, results will also include a serum/CSF ratio. It is recommended to have a concurrent fluid analysis performed on CSF at the time of SarcoFluor™ or NeoFluor™ testing to rule out blood contamination.
Why SarcoFluor™ and NeoFluor™ testing for EPM?
SarcoFluor™ and NeoFluor™ provide quantitative indication of EPM infections by providing actual titers and a likelihood ratio of disease correlating to the titer. UC Davis offers the only commercial platform that tests for both S. neurona and N. hughesi antibodies, ensuring more cost effective and complete screening from a single sample. Compared to other test formats, SarcoFluor ™ is more sensitive and specific for serum sample screening, decreasing the likelihood of false-positive results and reducing the need to obtain a CSF sample from an already medically-compromised horse.
NOTE: All laboratory requests and submissions must be made by a veterinarian, and results will only be released to a veterinarian. We cannot accept laboratory submissions directly from animal owners.
Forms
Sample submission instructions (PDF)
Sample Submission Form (fillable PDF)
Results Interpretation (PDF)
Pricing
Effective for samples received to the lab through December 1, 2025.
- IFAT panel (SarcoFluor™ and NeoFluor™) - $203/sample*
- SarcoFluor™ - $110/sample*
- NeoFluor™ - $110/sample*
- IFAT panel (SarcoFluor™ and NeoFluor™) for serum and CSF (includes serum/CSF ratio results) - $377/sample*
- CSF Fluid Analysis - $113/sample*
*Prices subject to change without notice. Updated 7/1/2025.
Include the following information with each submission:
- Check the test(s) requested on the sample submission form. ***Please note: If no tests are selected, an IFAT panel (SarcoFluor™ and NeoFluor™) will be run***
- Sample collection date and storage
- Shipment date
- Patient name, breed, sex, and age
- Patient’s owner name and address
- Clinic/hospital name and complete mailing address
- Veterinarian’s name
- Phone number, fax number and email
- Pertinent clinical history
Sample Processing & Shipping
- Blood Processing: Collect whole blood in a red-top tube (no additives). Allow the sample to clot completely, then centrifuge to separate the serum from the red blood cells. Transfer the serum to a new, clean, plain (no additive) labeled tube. Serum separator tubes are not recommended, as the separator becomes dislodged during shipping, allowing red cells and serum to mix. A handling fee will be assessed for unprocessed or improperly processed blood samples.
- CSF processing: Collect CSF in a clean red top tube. DO NOT spin CSF samples. If submitting samples for SarcoFluor™ and NeoFluor™ AND CSF Fluid analysis, please provide at least 2mL CSF.
- Store all samples at 4°C (refrigeration preferred to prevent mold or fungal growth), or room temperature if refrigeration is not available.
- Send all samples with a cold pack / ice pack.
- Ship overnight by FedEx (do not use USPS), Monday-Thursday only. Do not ship on Fridays, weekends or national holidays.
- Our FedEx Reduced Price Shipping Program is available for for veterinarians and veterinary clinics. FedEx is the preferred shipping method for overnight delivery. Please do not ship using USPS; USPS mail is delivered to a central campus location before being distributed to our labs, delaying transit time of sensitive laboratory samples.
- Ship to:
UC Davis VMTH
Clinical Laboratory Receiving, Rm 1033
1 Garrod Drive
Davis, CA 95616-8747
Turnaround times
- SarcoFluor™ and NeoFluor™ - 5-7 business days from date of sample receipt.
- CSF fluid analysis - 2 business days from date of sample receipt.
- Samples can be run “STAT,” for an additional fee; turnaround time for STAT samples is 1-3 business days from date of sample receipt for SarcoFluor™/NeoFluor™.
- All turnaround times assume there are no complications with testing; should complications occur, turnaround times may be delayed
Resources
- EPM Laboratory Diagnostic Flow Chart (pdf) (color, black and white)
- EPM Diagnostic Flow Chart (color, pdf)
Biological samples submitted to the VMTH Clinical Diagnostic Laboratories may be used for VMTH teaching and research purposes consistent with the mission of the University.
EPM and EPM testing references
"EPM Then and Now" The Modern Equine Vet. November 2021.
Molecular characterization of Sarcocystis neurona strains from opossums (Didelphis virginiana) and intermediate hosts from Central California. Rejmanek D, Miller MA, Grigg ME, Crosbie PR, Conrad PA. Vet Parasitol. 2010 May 28;170(1-2):20-9. doi: 10.1016/j.vetpar.2009.12.045. Epub 2010 Feb 11.
Utility of 2 immunological tests for antemortem diagnosis of equine protozoal myeloencephalitis (Sarcocystis neurona Infection) in naturally occurring cases. Johnson AL, Burton AJ, Sweeney RW. J Vet Intern Med. 2010 Sep-Oct;24(5):1184-9. doi: 10.1111/j.1939-1676.2010.0576.x. Epub 2010 Aug 12.
Prevalence and risk factors associated with Sarcocystis neurona infections in opossums (Didelphis virginiana) from central California. Rejmanek D, Vanwormer E, Miller MA, Mazet JA, Nichelason AE, Melli AC, Packham AE, Jessup DA, Conrad PA. Vet Parasitol. 2009 Dec 3;166(1-2):8-14. doi: 10.1016/j.vetpar.2009.08.013. Epub 2009 Aug 15.
Equine protozoal myeloencephalitis associated with neosporosis in 3 horses. Finno CJ, Aleman M, Pusterla N. J Vet Intern Med. 2007 Nov-Dec;21(6):1405-8.
Effects of blood contamination of cerebrospinal fluid on results of indirect fluorescent antibody tests for detection of antibodies against Sarcocystis neurona and Neospora hughesi. Finno CJ, Packham AE, David Wilson W, Gardner IA, Conrad PA, Pusterla N. J Vet Diagn Invest. 2007 May;19(3):286-9.
Indirect fluorescent antibody testing of cerebrospinal fluid for diagnosis of equine protozoal myeloencephalitis. Duarte PC, Ebel ED, Traub-Dargatz J, Wilson WD, Conrad PA, Gardner IA. Am J Vet Res. 2006 May;67(5):869-76.
Risk of transplacental transmission of Sarcocystis neurona and Neospora hughesi in California horses. Duarte PC, Conrad PA, Barr BC, Wilson WD, Ferraro GL, Packham AE, Carpenter TE, Gardner IA. J Parasitol. 2004 Dec;90(6):1345-51.
Evaluation and comparison of an indirect fluorescent antibody test for detection of antibodies to Sarcocystis neurona, using serum and cerebrospinal fluid of naturally and experimentally infected, and vaccinated horses. Duarte PC, Daft BM, Conrad PA, Packham AE, Saville WJ, MacKay RJ, Barr BC, Wilson WD, Ng T, Reed SM, Gardner IA. J Parasitol. 2004 Apr;90(2):379-86.
Comparison of a serum indirect fluorescent antibody test with two Western blot tests for the diagnosis of equine protozoal myeloencephalitis. Duarte PC, Daft BM, Conrad PA, Packham AE, Gardner IA. J Vet Diagn Invest. 2003 Jan;15(1):8-13.
Qualitative evaluation of selective tests for detection of Neospora hughesi antibodies in serum and cerebrospinal fluid of experimentally infected horses. Packham AE, Conrad PA, Wilson WD, Jeanes LV, Sverlow KW, Gardner IA, Daft BM, Marsh AE, Blagburn BL, Ferraro GL, Barr BC. J Parasitol. 2002 Dec;88(6):1239-46.
Neosporosis as a cause of equine protozoal myeloencephalitis. Marsh AE, Barr BC, Madigan J, Lakritz J, Nordhausen R, Conrad PA. J Am Vet Med Assoc. 1996 Dec 1;209(11):1907-13.

